42:271
MINERAL RESOURCES - course outline
ASHERN, Canada
Fall 2014
No text Notes on line
-------------------------------------------------------------------------------------------------
-
1st week (Oct. 24/25) Mineral Resources
Human Inventions
Classification
of metals
Mineral deposit types (14)
2nd week Non- metals & Industrial
Minerals Test
# 1
20 %
3rd week
Mineral collecting Test # 2 20
%
Fireworks
Gems / gemstones
4th week
Gem deposits Test # 3 20 %
Pegmatites Project
due 20 %
Project
Presentations
5th week Project
Presentations Final
20 %
Gems
in Manitoba / Canada
PROJECT: on 1 or more of chemical elements,
gems or mineral/rock deposits in
20% Manitoba or elsewhere. Very
important: include how useful the resource
is to our everyday life (example silicon is used in computers, rare
earth
metals are used in cellphones, etc)
Give exciting information about the subject that will be interesting for
students to learn about, and hopefully may trigger them to look for more
info
themselves
NOTES: Google search for “Ashern Mineral
Resources”
Notes to be posted 2 – 3 days before Friday’s class
lapis lazuli, Afghanistan
The "Rock" found north of Ashern: conglomerate from Canadian Shield brought in by glaciers
Visit to the Graymont Limestone / Quicklime Plant
The "Rock" found north of Ashern: conglomerate from Canadian Shield brought in by glaciers
Mineral Resources
42:271
Contents
•Periodic Table of
Elements
•Mineral Resources
•Standard
classification of metals
•Classification of
deposit types (14)
•Non-metals &
Industrial Minerals
•Mineral Collecting
•Fireworks
•Gems & Gemstones
•Gem deposits
•Gems in Canada
Periodic Table of
Elements
•Metals (copper)
•Non metals (oxygen)
•Alkali metals (sodium)
•Alkaline earth metals
(magnesium)
•Halogens (chlorine)
•Noble gases (helium)
Name of elements
•Discovered one by one
•Some from the
locality where they were found first (Yttrium from Ytterby, Sweden)
•Greek/ Latin roots in
most names
•Hydrogen= ydor,
hydro (water) + gene (creating)
•Helium is the
Greek word for the Sun, because it was discovered there by the spectrum
•Lithium= Greek word
for stone, because it comes from rocks not plants (like its neighbors on the
Table)
•Oxygen= acid creator
(erroneously thought)
•Chlorine= means
green-yellow
•Nickel= from Old Nick
meaning the Devil, because you get no copper. They thought the reddish mineral
would give Cu on smelting
•Molybdenum= meaning
“lead” or “a soft, black substance that can be used for writing”
•Antimony= “instead of
by itself” or not found by itself, always mixed with other minerals
•Iodine= the color of
violet (flower)
•Lanthanum= “easily
missed” because it is hidden among other minerals
•Gold= yellow
•Platinum= “small
silver” because it is white and usually found with gold
•Uranium= from planet
Uranus, discovered at about the same time as the element
The Elements
--------------------------------------- - - - - - - - - - -
currently in fashion :
Craton means continental basement
sections of uranium deposits with respect to the unconformity (boundary between two groups of rocks)
picture of "comb quartz", typical of U veins
Hoidas Lake, N. Sask.
50 km north of Uranium City
Most advanced REEs property in Canada (ready for mining)
Named after a WWII pilot who died in action
Contains a significant amount of heavy REEs, such as dysprosium- used in hybrid car components
Unfortunately, there is some U in the ore
•Arranged according to
atomic number (size of atom)
•From element # 1 to
element # 26 (Iron) manufactured by our Sun (the star)
•All higher than # 26
manufactured by supernova explosion of pre-existing stars
Therefore, our solar
system is second generation star system (attractive to aliens?)
The Periodic Table
•Tabular arrangement
of elements
•Atomic number is # of
protons in the nucleus
•Rows called periods,
columns are groups
•18 columns, 7 rows
with a double row below
•4 rectangular blocks:
s-block to the left, p-block to the right, d-block in the middle & f-block
below
•The table
incorporates “recurring trends”, like similar properties
•Table can also
predict properties of new elements
•Atomic number is # of
protons in the nucleus- equal to # of electrons
•A new row (period) is
created when the atom has a new electron shell & has its first electron
•Elements with the
same # of electrons in a particular shell occupy the same column
•Elements with similar
properties belong to the same group
•Today, 114 confirmed
elements
•Only 98 elements
occur naturally
•There are 18 columns
or groups
Can change one element
to the next
•With a higher atomic
number
•Simply by giving it
energy
•If unstable, it will
give off this extra energy – doing some useful work – and change back to its
original
•Example from
“Jerusalem Dome of the Rock UFO”
•Flash is when it
changes into a higher element, then gives off its energy by climbing up fast
while it changes back to its earlier form – the element created is probably
element 116 which is unstable
Electronic equipment
we use
•Necessary to have
good conductors (metals)
•Need special
properties of conductors
•Many of these are
provided by the REEs
•Rare Earth Elements:
China has the bulk of them, but others may have them too
•Need exploration to
find them
Where to find
information
•Textbooks
•Unfortunately, the
textbook for this course is out of print, new one not ready
Info on the web
•Mineral Resource
Classification: Wikipedia
•Ore Genesis :
Wikipedia
•Hydrothermal
circulation: Wikipedia
•Hydrothermal
synthesis: Wikipedia
•Volcanogenic massive
sulfide ore deposit: Wikipedia
•Seafloor massive
sulfide deposits: Wikipedia
Info on elements for
project
•Look under name of
element / metal
•Look under deposit of
that element / metal
•Find something you
like or you want to know more about (such as the gems of Manitoba!)
•Make a presentation
and/or send by email
Where the metals come
from?
•Every rock you pick
has traces of metals, but you can’t see them – in trace amounts
•These metals can be
absorbed by hot water solutions going through the rocks
•The solutions will
also have other solvents like sulfur that can dissolve other metals
•When conditions
change along the way the metals can be deposited – they precipitate out
Examples of changing
conditions
•Change in temp. or
pressure
•Change in rock type
Metals
•Their abundance in
rocks of various types has been measured
•In order for them to
become “ore” nature has to collect them in some place or trap
•This concentration
over millions of years of time has created ore deposits
•The concentration
factors of metals varies quite a lot
Table: Concentration of
metals into ore
•
abundance
concentration factor
•Al
8.2%
4
•Fe 5.6
%
9
•Cu 55
ppm
180
•Zn 70
ppm
700
•Au 4
ppb
1250
•Pt
5 ppb
1000
Magma
•Dissolves surrounding
rocks
•Within a liquid mass,
some metals can accumulate if they are heavier, for example
•These “heavies”
accumulate at the bottom of magma chamber and can solidify to form a layered
deposit when magma cools down, for example, chromium, platinum
Human history and
resources
•Stone Age
•Copper Age – but Cu
is soft
•Bronze Age 3,000 BC – Cu + Sn (tin)
•Iron Age
1,200 BC
•Many new discoveries
since industrialization ~ 200 years ago
Mineral Resources
•Resources (available
if feasible) v. reserves (ready to mine)
•Material of economic
interest. Needs to be calculated, usually after drilling. Then, called “ore”
•Theories on how
materials form in the Earth’s crust: “ore genesis”
•Material has a
source, usually magma, then it is transported and deposited in a suitable
environment (trap)
Ore genesis processes
•Inside the Earth:
magmatic, hydrothermal, metamorphic
•On the surface of the
Earth (example, gold nuggets in a river)
Concentration of
metals
•One can get a clue of
the mineralizing solutions from the so-called “fluid inclusions” or remains of
those fluids trapped in the rocks
Fluid Inclusions
•4 common types
•Aqueous: liquid +
minor vapor
•Aqueous: vapor +
minor liquid
•Aqueous; liquid,
vapor, halite, anhydrite
•CO2 – bearing
Compare them to our
own blood
•Which passes through
our body bringing nourishment, oxygen, etc & removing other substances
•(blood is a solution
identical to the sea water)
•Most metal deposits
are sulfides
•But metal sulfides
are extremely insoluble in pure hot water
•Evidence comes from
fluid inclusions: small samples of fluid trapped in minerals
•Inclusions contain a
lot of Na, Ca, Mg, Cl & S and metals
•Metals transported as
soluble compounds, such as PbCl2 + H2S = PbS + 2HCl
example
•Galena (PbS) precipitated
when the lead compound comes in contact with hydrogen sulfide gas
Standard
classification of metals
Common ores
•Iron
•Lead-zinc-silver
•Gold
•Platinum
•Nickel
•Copper
•Uranium
•Titanium and zirconium
•Tin, tungsten and
molybdenum
•Rare earths, niobium,
tantalum, lithium
•Phosphate
•Vanadium
•Gems
•Industrial minerals
(sand, gravel, diatomaceous earth, silica sand)
--------------------------------------- - - - - - - - - - -
currently in fashion :
Rare Earths (REEs)
17
reference: geology.com
Yttrium +
•The 15 Lanthanoids
Scandium
•Also is found
together with the others and listed as a REE
All REEs
•Have similar
properties
•That is why they are
found together
•They are all metals
•They are all sold as
oxides
Uses
•In computer memory
•DVDs
•Rechargeable
batteries
•Cell phones
•Catalytic converters
•Magnets
•Fluorescent lighting
•etc
Batteries
•For electric and hybrid cars
Catalysts, phosphors,
polishing compounds
•These are used for
air pollution control
•Illuminate screens on
electronic devices
•Polishing on high
quality glass
The Military
•Night vision goggles
•Precision-guided
weapons
•GPS equipment
•Make hard alloys for
armored vehicles
•Projectiles that
shatter in thousands of sharp fragments
Are they rare?
•Not really
•However, they are
difficult to find in concentrations high enough to be mined
History
•Before 1950’s no
demand
•Found in heavy sands
of Brazil & India
•Then, in South
Africa’s monazite sands
•Later on, in a
California carbonatite
•Demand exploded with the
coming of color TV in the 1960’s
•China eventually
became the biggest producer
Exploration
•In high gear world
wide
•It is believed that
China, who produces 90 % of world’s supply, actually only has 50 % of world’s
resources
•The rest 50 % are
spread in other countries
•So, start looking…
Where to find them
•Alkaline igneous
rocks & magmas
•Rare Earth placer
deposits
•Residual Rare Earth
deposits
•Rare Earth elements
in pegmatites
•Other Rare Earth
deposit types
•Mineral processing is
challenging
Alkaline igneous rocks
•Form from cooling of
magma from the mantle
•Process not fully
understood
•Rare magma unusually
enriched in Zr, Nb, Sr, Ba, Li & the REEs
•More changes as magma
ascends through various rocks on the way up
•Great variation in composition
of alkaline rocks and their metal deposits
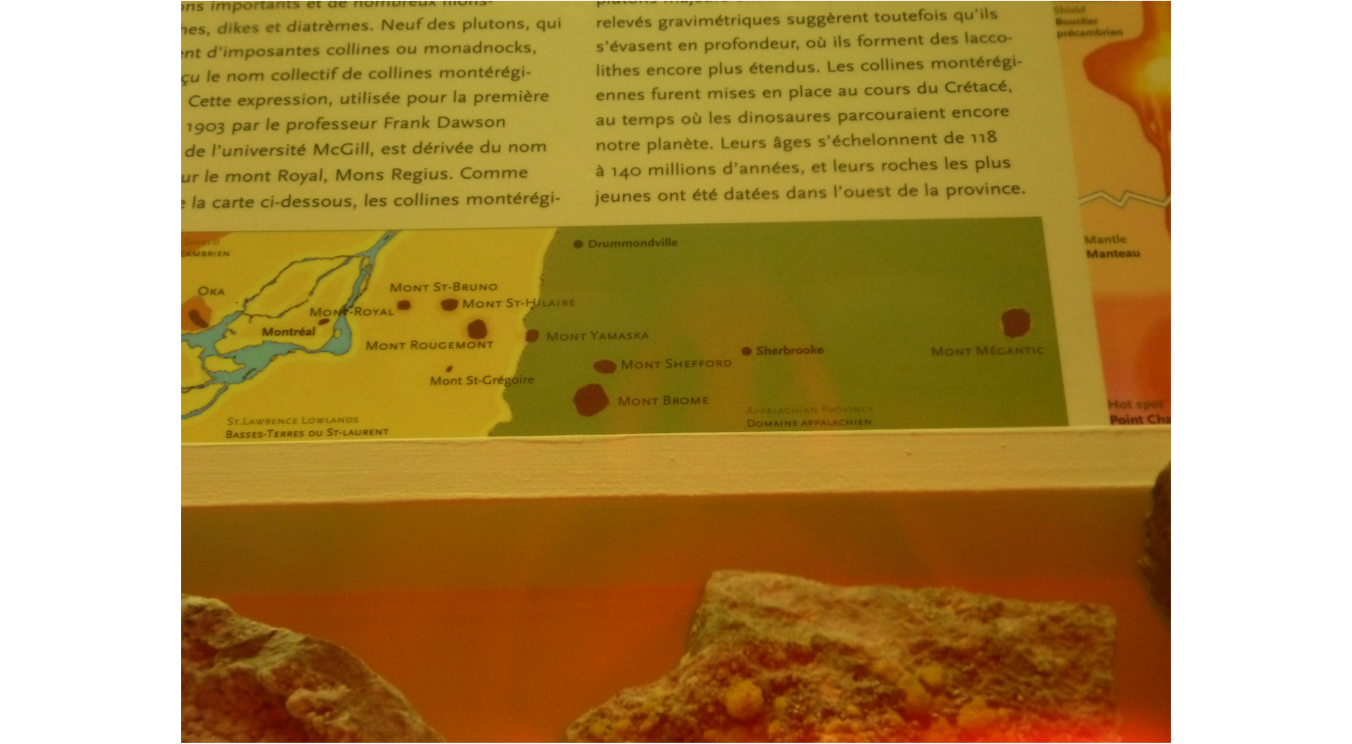
Example of alkaline
igneous rocks
•Montreal or Mount
Royal
•The hill was a volcano
•So are numerous hills
in the vicinity, all lined up. Formed by the movement of the plate over a hot
spot
•They have been
studied, but their REEs contents not calculated yet
Rare Earth placer
deposits
•Weathering of rocks
of all kinds yields sediments deposited in rivers, shores & deltas
•Denser minerals such
as gold accumulate into placers, also monazite, ilmenite, cassiterite, etc
Residual Rare Earth
deposits
•In tropical areas,
rocks are deeply weathered to form laterite, an Fe & Al-rich soil
•The process of soil
formation concentrates heavy minerals over the underlying bedrock
REEs in pegmatites
•Are generally small,
not for commercial production
Other Rare Earth
deposit types
• deposit, Australia
is unique
•Fe oxide, Cu-Au with
large amounts of Rare Earths and uranium
•However, no method to
recover these REEs
•Karst bauxites are
enriched in REEs
Mineral processing: a
challenge
•The ores of REEs are
complex and commonly radioactive
•To separate one metal
from one mineral (Cu from chalcopyrite) is simple and easy
•Very challenging to separate
2 or more metals from a single mineral or multiple minerals
•Minerals containing
many REEs must be separated and refined. The presence of radioactivity makes it
difficult to mine due to to many regulations imposed
-------------------- - - - - - - - - - - - - ------------ - -
Iron (Fe)
•Mostly magnetite in
ancient sediments accumulated on the ocean floor in an oxygen poor atmosphere
and acidic water
•Weathering converts
magnetite into hematite, much easier to process
•Some deposits within
the Pilbara region of W. Australia formed as placer deposits
•They formed by
accumulation of hematite gravels called pisolites, which form channel-iron
deposits
Iron Formations
•Found in Proterozoic
rocks (Precambrian) worldwide
•Large deposits in
Ontario & Quebec
•Small occurrences
throughout Manitoba – example, near Bissett
•Big production in
Michigan (cell phones may not work in some places due to abundant magnetite deposits)
pictures of Iron Formation : magnetite layers are black and chert layers can be red or other colours. Red colour would be similar to the colour of seawater at the time!
pictures of Iron Formation : magnetite layers are black and chert layers can be red or other colours. Red colour would be similar to the colour of seawater at the time!
Lead-Zinc –Silver
•Form by the discharge
of deep sedimentary brine onto the sea floor (SEDEX), or by replacement of
limestone, some associated with volcanoes.
•Vast majority of
SEDEX deposits are of Proterozoic age, some are of Jurassic age.
•The carbonate
replacement type deposits form by degradation of limestone by hydrocarbons
which are thought to be important for transporting lead
•The minerals are
galena & sphalerite. Silver is found in inclusions within galena
•Silver-rich galena
will have a striped appearance (see hand samples)
Gold (Au)
•Form in a very wide
variety
•Primary or placer or
residual
•Plate tectonics
generates gold deposits
•Primary are lode or
intrusion-related
•Lodes associated with
orogeny or other collision events
•Most lode deposits
sourced from metamorphic rocks
•Form by dehydration
of basalt during metamorphism
•Gold transported up
faults by hydrothermal waters and deposited when the water cools too much to
retain gold in solution
•Lode deposits are
high-grade, thin, vein or fault-hosted. Usually in quartz veins or reefs
•Usually hosted in
basalt or in sediments known as turbidites, although when in faults that may be
in granite
•Intrusive-related
gold hosted in granite, porphyry or dikes. May also contain copper, tin,
tungsten, etc
•These deposits rely
on gold existing in the fluids associated with the magma
•Placer deposits form
via gravity with gold sinking into a trap or bends in rivers
Malartic mine,
Quebec –largest in Canada (probably)
west of the pit above, the old East Malartic mine - where I worked. Notice the shaft has an inclined component - that was the shaft going down into the rock
Gold in a chert
horizon – picture
Red Lake mine: rock has 220 oz of gold! worth 330,000 $ at today's prices- sorry out of focus!
Large nuggets in a rock mine: Red Lake, Cadillac mine, Que. & NW Ontario/Man. border
picture from Red Lake
Red Lake mine: rock has 220 oz of gold! worth 330,000 $ at today's prices- sorry out of focus!
Laterite gold
•Form after prolonged
weathering of primary gold deposits
Now rock made up of red iron oxide, clays and a bit of native gold
Now rock made up of red iron oxide, clays and a bit of native gold
Uses of Au
•For 70 years it was a
standard treatment for rheumatoid arthritis to inject a liquid suspension of
gold, which acts like an anti-inflammatory – no one knows why or how
•Windows coated with
Au to help reflect the sun in the summer and retain heat in winter
•About 20 % of
decorative gold is in the thread of Indian saris
Toronto’s Royal Bank
Plaza
•Two 41-and- 26 storey
towers
•Has 14,000 windows
•All have very thin
coating of 24-carat gold leaf
•2,500 ounces (70.8
kg) of gold worth over 1 million $
Can you change metal
into Au?
•Soviet nuclear
reactors transformed some lead nuclei into gold
Gold in history
•World production from
ancient times to now is about 165,000 tons
•A lot is reused
•Biggest source of
gold is in the ocean water, but you can’t recover it
•Alexander inherited
5,000 tons of gold –the bulk of the gold produced prior to zero year -when he
conquered Persia (it brought its gold from Uzbekistan / Tajikistan, Persia had
none)
Alexander's funeral procession - all made up of gold supposedly
Alexander's funeral procession - all made up of gold supposedly
•Egypt has many gold
treasures, but the country has no gold – it brought it from Sudan
Pharaoh sarcophagus
Pharaoh sarcophagus
•California “started”
with the Gold Rush, later a “Yukon Rush” in the Klondike area (depicted in
movies of Charlie Chaplin)
•Latest production
comes from the shallow “black smokers” of the Pacific ocean
Platinum (Pt)
•Pt and palladium are
generally found within ultramafic rocks
•Source of the metals
is rocks that have enough sulfur to form a sulfide mineral while the magma is
still liquid
•The sulfide mineral
(pyrite, etc) gains platinum by mixing with the bulk of the magma because Pt is
chalcophile (it likes Cu) and is concentrated in sulfides
•Sulfide phases only
form in ultramafic magmas when the magma reaches sulfur saturation.
•To achieve sulfur
saturation magma must get contaminated with sulfur-rich wallrock or mixed with
other magma
•Often, Pt is
associated with Ni, Cu, Cr and Co deposits
Most of world’s
production
•Comes from the
Merensky Reef in South Africa, a continuous layer that formed by fractional
crystallization within one magma chamber
•Minor production
comes from Thompson, Man. and Norilsk, Siberia
•Pt used in seat belts
– excellent conductor
Nickel (Ni)
•Two types, as sulfide
or laterite
•Sulfide form the same
way as Pt deposits
•Ni is a chalcophile
element which prefers sulfides, so an ultramafic or mafic rock which has a
sulfide phase in the magma may form nickel sulfides.
•Best Ni deposits
accumulate at the base of komatiite lavas (Mg-rich)
Thompson / Voisey Bay
deposits
•Subvolcanic sills
host Ni sulfide deposits formed by deposition of sulfides near the feeder vent.
Sulfide was accumulated near the vent due to the less of magma velocity at the
vent interface
- see hand samples with pyrrhotite
Sudbury deposits
•The Sudbury Basin or
the Sudbury Nickel Irruptive is a major geologic structure in Ontario
•It is the second
largest impact crater on Earth as well as one of the oldest (1.8 b.y. old)
•The large impact
crater filled with magma rich in Ni, Cu, Pt, Pd, Au & other metals
Norilsk deposits,
Siberia
•Largest Ni-Cu-Pd
deposits in the world
•Deposit formed 250
m.y. ago during the eruption of the Siberian Trap Igneous Province (STIP). The
STIP erupted over I million cubic km of lava, a large portion of it through a
series of flat-lying conduits
•The ore formed when
the erupting magma became saturated in sulfur, forming globules of sulfides.
These sulfides were then “washed” by the continuing torrent of erupting magma
& upgraded their tenor with Ni, Cu, Pt & Pd
Ni laterite deposits
•Similar to formation
of gold laterites except that ultramafic or mafic rocks are required
•Very large
olivine-bearing ultramafic intrusions
Uses of Ni
•The Loonie: bronze
plated on pure Ni (7 g wt)
•In CDs, cell phones,
batteries, electric shavers, golf clubs, credit cards, etc
Copper (Cu)
•Either formed within
sedimentary rocks or in igneous rocks
•Most major copper
deposits within the granitic porphyry copper style
•Sedimentary Cu forms
in ocean basins. Cu is precipitated by brine from deeply buried sediment
(similar to SEDEX zinc)
More information under "Porphyry Copper"
Uranium (U)
•Source is radioactive
granites when certain minerals like monazite are leached during hydrothermal
activity or by circulation of groundwater
•U is brought into
solution by acidic conditions & is deposited when this acidity is
neutralized
•Generally this occurs
in carbon-bearing sediments within an unconformity
Precambrian Shield
Craton means continental basement
picture of "comb quartz", typical of U veins
hand samples of U from Saskatchewan in class
•U also found in coal
and in all granites
•Radon gas creates a
problem in U mining
•40% of world U in the
Olympic Dam deposit in Australia. U in granite & porphyry
Uranium City area,
Sask.
•Production from
1960’s until 1982
•Newer high grade
deposits discovered under the Athabasca sandstone Basin in N. Sask. have huge
reserves & tremendous potential
•Unconformity with
underlying basement is the target wherever graphite sediments are present
Titanium &
Zirconium (Ti, Zr)
•Mostly found as
mineral sands
•By accumulation of
heavy minerals in beaches (like placer)
•Titanium as ilmenite,
rutile & leucoxene
•Zirconium as zircon
•Thorium in monazite
•All found in granite.
After erosion & transportation by rivers into beaches
Zircon: yellow crystals in granite, contain U, Th, used as a opacifier (making things opaque) in ceramics
Zircon: yellow crystals in granite, contain U, Th, used as a opacifier (making things opaque) in ceramics
Tin, tungsten &
Molybdenum
•Form in certain type
of granites
•Skarn deposits form
by reaction of mineralized fluids with the rocks – such as limestone
-surrounding the granite
Tin: used in tin cans, making bronze, now produced in Malysia from alluvial deposits
Molybdenum: found with Cu in porphyry deposits. Used in making steel
Tungsten: heavy metal, rare in nature. Used to steel, carbide, lamp filaments
Molybdenum: found with Cu in porphyry deposits. Used in making steel
Tungsten: heavy metal, rare in nature. Used to steel, carbide, lamp filaments
Rare Earths, Niobium,
Tantalum, Lithium
•The majority of REEs
+tantalum & lithium- found within pegmatite
•Probable origin by
metamorphism & igneous activity
•Lithium as spodumene
& lepidolite
•Carbonatite
intrusions an important source of these elements
Example:
Bernic Lake, Manitoba: Tanco mine
•Lithium
•Cesium - 80% of world's production
•Tantalum
•Columbium
•Beryllium
Hoidas Lake, N. Sask.
50 km north of Uranium City
Most advanced REEs property in Canada (ready for mining)
Named after a WWII pilot who died in action
Contains a significant amount of heavy REEs, such as dysprosium- used in hybrid car components
Unfortunately, there is some U in the ore
Phosphate
•Used as fertilizers
•Immense quantities in
sedimentary rocks from the Proterozoic age to now
•The source of P is
the skeletons of marine animals
•Another source is
intrusions like nepheline syenite & carbonatites
•The P is in apatite
& monazite
Vanadium (V)
•Found in blood cells
of vanabins in the sea
•Biological processes
responsible for the deposits
•Also found in crude
oil & oil sands
used to make steel
used to make steel
Tellurium (means from
the earth) has accomplished:
•Cheaper photovoltaics
•More robust CDs
•Spectacular pictures
from the Hubble telescope
•However, when you
work with the metal it enters body and for many months your sweat, urine has a
garlic smell!
Classification of
deposit types
ORE DEPOSIT MODELS
•14 types
1. Layered mafic-ultramafic
intrusions
•Intrusions with Ni-Cu
sulfides at the base
•Fractional
crystallization with density stratification
•Chromite-rich layers
(Cr) – Bird River, Man.
•Other metals: Pt
group elements
- Cross Lake: magnetite with Ti
- Cross Lake: magnetite with Ti
2. Carbonatite
Alkaline intrusions: REEs
•Igneous intrusions
made up of calcite-dolomite-ankerite with apatite & magnetite
•Alkaline (Na and K
rich) volcanic rocks
•Related to
continental rifting & alkaline volcanism
•REEs: Ce, Y, La, Sm, Eu
Example from Montreal
area
Mont St. Hilaire, Que.
One of the Monteregian hills
Has 3 distinct intrusions: gabbro, nepheline syenite & pegmatites
Famous mineral locality- rare & exotic
366 minerals, 50 of which are “endemic” (type location)
Most Fe-rich biotite in the world!
picture: large crystals
granite and metal zoning
Another classification
Abundant Metals
Al
Fe
Mg
Ti
Silicon Valley, California
South part of San Fransisco
Silicon chip semiconductor used in high technology operations (computers)
1/3 of venture capital in the USA raised here
Best place for high tech jobs in the USA
Many universities
Specialists recruited/come from around the world
Sulfur
Bright yellow crystals at room temperature
Can react as either an oxidant or reducing
Common as sulfide of metals or sulphate
Brimstone (burn stone) used as fumigant
Rotten egg smell (hydrogen sulfide & SO2)
Responsible for smell in cabbage, broccoli, garlic, onion & skunk
In proteins, aminoacids
In Canada, byproduct of natural gas, petroleum & Tar Sands
NOTE: for the remainder of the course search Google under "Ashern Gems"
Mont St. Hilaire, Que.
One of the Monteregian hills
Has 3 distinct intrusions: gabbro, nepheline syenite & pegmatites
Famous mineral locality- rare & exotic
366 minerals, 50 of which are “endemic” (type location)
Most Fe-rich biotite in the world!
carletonite: type locality
purple syenite complex
Monteregian Hills, Montreal area
3. Porphyry Copper intrusions
Porphyritic granitic rocks with a network of chalcopyrite veins
At collision of plate boundaries of Andean type or island arcs – mountains today
Alteration zones with inner potassic, outer sericite & peripheral chlorite-epidote
Fluids from the pluton followed by later lower temp. fluids leaching Cu & S from the magma & surrounding rocks
Porphyry copper deposits
cross section
Alteration zones
example: Cerro Colorado, Panama
The deposit is the whole mountain!
The paths are for exploring & moving the drill
platforms are for drilling
steep terrain
paths are 100 m apart vertically
old volcano in the distance
my map: red is porphyry, green is granite
3D of deposit
Cerro Colorado Cu-Mo deposit
Cu –Mo not visible on surface, they have been oxidized and removed from near surface
The “country rock” is basalt that deposited on the ocean floor
Two main intrusions: a porphyry (red on map) and granite (green). Both are mineralized with a network of veins carrying chalcopyrite (yellow) and molybdenite (bluish) and minor pyrite
Alteration zones
The distribution of the Cu-Mo follows the alteration pattern
Higher grades are in the potassic & sericite zones and lower grades in the chlorite/calcite/epidote zone
There seem to be two separate deposits that formed by the two different intrusions of the area (porphyry & granite)
One of the biggest in the world with reserves over 3 b.tons (reserves calculated down to sea level). Highest elevation is 1,600 m
samples of porphyry type mineralization in class
made headlines in the Northern Miner
4. Complex Pegmatites
Late stage of granitic intrusions that have undergone fractional crystallization
The last fraction of the magma would be rich in water & incompatible elements that could not fit into the earlier formed minerals
Li, Be,B,F, Rb, Cs, REE, Th, U, Ti, Zr, Hf, Sn,Nb
made headlines in the Northern Miner
The deposit does not reach surface
Late stage of granitic intrusions that have undergone fractional crystallization
The last fraction of the magma would be rich in water & incompatible elements that could not fit into the earlier formed minerals
Li, Be,B,F, Rb, Cs, REE, Th, U, Ti, Zr, Hf, Sn,Nb
picture: large crystals
5. Massive sulfides – Cyprus type
Massive pyrite with Cu & Zn sulfides in pillow basalt (ophiolite)
The ophiolite represent portions of the ocean crust formed at mid-ocean ridges. Seawater circulates through fractured basalt & heated to 400’ C, while metals are leached from basalt and deposited on ocean floor
Part of ocean floor is preserved by thrusting onto adjacent continent
6. Cu-Zn felsic volcanic deposits
Cu & Zn in marine volcanic rocks
In island arcs related to subduction zones
Examples in Japan, Ontario, Quebec
7. Uranium clastic sedimentary deposits
In permeable sandstone that cemented later
U from volcanoes
8. Mississippi Valley carbonate Pb-Zn
Galena, sphalerite, chalcopyrite in porous rocks related to reefs
In dolomite & breccias
Epigenetic ore (after the rock formed)
Cambrian to Carboniferous
Metals from basement. Low-temperature fluids & highly saline with sulfur
Example: Pine Point, NWT
Open pit operation
Pine Point deposits
Galena
Samples showing
Galena – sphalerite with calcite/dolomite
Pockets of sulfur
Pockets of bitumen
The host rock -limestone/dolomite- has been altered by the mineralizing fluids
When you hit the rock with the hammer it smells gasoline (it contains petroleum/oil from remains of marine animals like corals)
9. Banded Iron Formations
ayered with chert
Great lateral extent
Deposited in shallow water (shelf)
Mostly Proterozoic in age
High dissolved content of SiO2 and Fe+2 in seawater from leaching sea volcanoes
10. Aluminum Ore- bauxite
Weathering over any aluminum-rich rock (granite)
Mainly Cenozoic
Clay minerals associated with hematite, limonite
Tropical climate promotes breakdown of feldspars into clays rich in Al. Silica, Iron & other solubles leached out
ALCAN: Canadian co. has smelter in Kitimat, BC
11. Placer deposits: Au, Diamonds & heavy minerals
Pt, Au, diamonds, magnetite, chromite, ilmenite, cassiterite, zircon
Concentration in low-energy areas of stream flow (inner curves of meanders)
World’s largest nugget
12. Tin vein –Cornwall type
Quartz-cassiterite (+/- Tungsten, base metals) veins associated with granite
Intrusions in fold belts or collision of continents
Similarities with porphyry coppers with alteration patterns
Oxides within granite and outer sulfides of Cu, Zn, Pb, antimony
Cornwall, U.K.
Southwest corner of England
People are Celts that have worked the tin mines for ~ 2,500 years
Production from here responsible for Bronze Age
Ancient Greek mariners called the British Isles “the Cassiterides” which means the Tin Islands
In the 1800’s more than 300 mines, declined slowly, none left today
The miners moved to California, S.Africa & Australia. Some into Ireland where they call them “Tinkers” prob. because they worked with tin. The Irish don’t like them, treat them like the gypsies. Their graves are spectacular & spend a fortune on them – see pictures
The Cornish recently became semi-independent and have their own language (Kernow)
Local products: Cornish pasties, clotted cream
Tinkers’ graves
Granite (red), killas (brown), veins (yellow)
Cornish words
Killas is their word for “country rocks” or local rocks
Elvans is how they call the dikes or small intrusions like walls
Mine names usually start with “Wheal”, like Wheal Jane is one of them
granite and metal zoning
old mines
Tin ore
Tin veins
13. Mercury – Almaden type (Spain)
Stratabound disseminated cinnabar (HgS) & native mercury in volcaniclastic sedimentary rock
Permeable sedimentary rocks near volcanic center with faulting
No metamorphism or granite intrusion
Modern hot springs are associated with Hg deposits suggesting ancient deposits formed by circulating hot fluids along faults, with the volcanic rocks acting as the source of the Hg
Low solubility of Hg compounds requires very large amounts of fluid. Hot springs may provide such an environment
Hg has a low boiling point, it may be transported as a vapor & deposited as pure (native) Hg
Mercury (quicksilver) ore
14. Nickel laterite deposits
Deep weathering of ultramafic rocks in warm humid climate
Enriched in Ni, Co, Cr
Alteration from top down, red & yellow soil (limonite), saprolite, altered ultramafic rocks
Abundant Metals
Al
Fe
Mg
Ti
Scarce Metals
Base metals: Zn, Cu, Pb, Sn, Cd, Hg
Ferroalloys : V, Cr, Ni, Co, Mo, W, Mn
Precious metals: Au, Ag
Platinum group metals: Pt, Pd
Rare Earth metals
Lightest & Heaviest
Lithium v. Uranium
Non-metals & Industrial minerals
Limestone, clays, sand, gravel, diatomite, silica, barite, gypsum, talc, perlite, zeolites, etc
Used in construction, ceramics, paints, electronics, filtration, plastics, glass, detergents, paper, agriculture, etc
Fertilizers
Nitrogen
Phosphorus
Potassium
Sulfur
Evaporites
Potash
Salt (halite)
Gypsum & Anhydrite
Borates
Sodium sulfate
Potash
~ 30 % of world’s supply from Sask.
Reserves for centuries
One danger: flooding by water
1 km deep layers within sedimentary rocks
Devonian age deposited in a cut-off portion of the ocean
Salt (halite)
Groundwater down to about a 200 m depth is fresh
Below that it is saline (brine)
An anomaly (man-made or not) can bring saline water higher up – like south of Winnipeg
Salt in Manitoba
Salts in the Prairies
Gypsum
Making gyprock (drywall, plasterboard)
Raw gypsum is heated and water driven off, then partially re-hydrated, wet product then sandwiched between two heavy sheets of paper and dried
Barite (means “heavy”) & usage
Has a SG of 4.5 (~ double of ordinary rock)
In drilling muds
In pigments, paper industry
In playing cards (denser) – easier to “deal”
Blocks X rays & gamma rays
Silica sand (mostly quartz)
Many, many uses
Found in deposits of sand in non-tropical areas
Making glass, concrete, sandblasting, industrial casting, etc
Fine quartz, however, is dangerous if inhaled (silicosis)
Silicon Valley, California
South part of San Fransisco
Silicon chip semiconductor used in high technology operations (computers)
1/3 of venture capital in the USA raised here
Best place for high tech jobs in the USA
Many universities
Specialists recruited/come from around the world
The many uses of Fluorite
Making glasses, enamel & ceramics
Non-stick Teflon cookware
In production of steel as flux –removes sulfur
Has exceptional optical clarity & used as lenses that show extremely sharp image- for cameras, microscopes & telescopes
Sulfur
Bright yellow crystals at room temperature
Can react as either an oxidant or reducing
Common as sulfide of metals or sulphate
Brimstone (burn stone) used as fumigant
Rotten egg smell (hydrogen sulfide & SO2)
Responsible for smell in cabbage, broccoli, garlic, onion & skunk
In proteins, aminoacids
In Canada, byproduct of natural gas, petroleum & Tar Sands
Carbon
All Life is carbon based (organic chemistry)
Element known since ancient times
Diamond & graphite (good conductor)
Found in limestone, CO2 gas & methane CH3
Forms more compounds than any other element, more than 10 million
Has no melting point, it sublimes
Forms carbides (extremely hard) with tungsten
In order to form Life, carbon needs to get scattered in space as dust from supernova explosions and then later incorporated into a 2nd or 3rd generation star (our Solar System is 3rd)
Graphite
Both USA & EU declared graphite as a strategic mineral
Important in high technology & green energy
80% produced in China, however, reserves are declining
Many uses
Refractories
Batteries
Brake linings
Steel making
Laptop computers
Manganese
World’s healthiest foods rich in Mn (cloves, oats, rye, spinach, etc)
We need small amounts in diet
Primary use in making steel
Not found in native (pure) state, usually associated with iron
Name from Magnesia, Greece, confused with magnetite, but Mn NOT magnetic
Found on the ocean floor as nodules, but too difficult to mine, est. 500 billion tons
Can have neurological problems if too much in the body from drinking water, etc
Used as additive to gasoline (instead of lead)
Selenium (=moon)
Found with sulfur
Used in electronics, glassmaking & pigments
Used in medical pills (thyroid problems) & infant formula
Many thyroid problems in Europe after the Yugoslavia war of 2001 when U bombs were used for first time
Neon
One of the noble gases
Inert, not reactive gas
5th most abundant element in the Universe after H, He, C, O
Has a notably bright red light
Used in advertizing signs
Aggregates
and & gravel
Crushed stone
Cement
Dimension stone
Limestone – common in Manitoba
Granite – expensive, mostly as counter top
Gneiss – expensive, mostly as counter top
Peridotite – green with marble-like finish, Winnipeg Convention Center
Granite porphyry – Regina Government buildings
Limestone / dolomite/cement
Quicklime or calcium oxide (CaO)
Calcium oxide is obtained by heating limestone at more than 825 degrees C to liberate carbon dioxide gas
If left alone it can grab CO2 from the air to form limestone again
If quicklime is heated to 2,400’C it emits an intense glow, called limelight; was used in old theatres before the advent of electricity
Quicklime is the main ingredient to make cement
(Portland) cement
Bricklayer Joseph Aspdin of Leeds, England first made cement early in 19th cent. by burning powdered limestone and clay in his kitchen stove
To make cement today we use limestone, clay, silica sand and iron ore. They are heated at high temp. and form a rock-like substance that is ground into the extremely fine powder we call cement
cement
Cement is mixed with water to make mortar
Or mixed with sand, gravel and water to make concrete
Other uses of CaO some info from visit to Limestone Quarry
To neutralize acidity in base metal mines, like in Flin Flon who are treating sulfide-rich ore (acidic)
With water it gives out a lot of energy, so it can be used for on-the-spot food warming
Farming to neutralize acidity in soil
In pulp mills
In petroleum industry
Rock/Mineral collecting in Canada
130 clubs
Bancroft, Ontario: 1 of only 3 in the world, where ancient conditions created nearly every known volcanic & metamorphic rock & mineral
Mont-St-Hilaire, E of Montreal – among the top 10 in the world sporting 366 minerals
Bay of Fundy: endless varieties of agate also zeolite & amethyst
Thunder Bay: abundant amethyst, even the highways are purple in color. Also agate
Souris pit: agates & petrified wood
Bancroft, Ont.
Major attraction is the mineral-rich pegmatites. They are like magma that could not come to the surface as lava, instead it cooled at depth slowly
Rose quartz (color due to Al?) is prominent
Apatite, sodalite, rare earths, uraninite, zircon
Marble was used across the country
Feldspars, a major product
Part of ceramics, enamel, pottery
To make paints, soaps, roofing, tiles
Its highest grade to make false teeth
Its lowest grade in whipping up stucco
The elements in FIREWORKS
Ancient Chinese used a mixture of sulfur, saltpeter and charcoal that was very flammable and would explode if put in a miniature amount of space. That was 7th cent.
However, at the same time the “Greek Fire” was invented in 672 AD. Although its composition was a state secret and remained so, it must have been a mixture of things like pine resin, naphtha, quicklime, sulfur & niter
The “Greek Fire” – in Greek, “liquid fire”
Clay grenades filled with Greek Fire
Anatomy of fireworks: many parts
A launching tube, 3 X the length of the fireworks shells, but exactly the same diameter
When the gunpowder ( 75% saltpeter, 15% charcoal, 10 % sulfur) burns, the heat and gas are trapped & force their way to the surface until an explosion happens & they become airborne
A fuse
Ingredients
An oxidizer, a reducing agent, a coloring agent, binders and regulators
Oxidizers (chlorate) produce the oxygen to burn the mixture
Reducing agents burn the oxygen to make hot gases
Binders hold the mixture in a lump
Incandescence & luminescence
The light produced from heat is incandescence
Ti & Al used to increase the temp. & cause the firework to burn brighter
Light produced by energy sources other than heat is luminescence
Energy absorbed by an electron will cause it to be excited. When the electron’s energy is lowered it is released as light
Colors
Cu creates blue
Al to give silver & white colors & sparks
Zn makes smoke clouds
Ba for green, Sr, Li for red, Mg for white, Na for gold & yellow
Cl brightens the colors
Fluorescent Minerals
UV radiation generated by a uv lamp
About 15% of minerals respond well to longwave uv energy
Named after fluorite, the 1st mineral to show it
Longwave LED (Light-emitting Diode) lights are popular, they generate less heat than ordinary ones
You can’t look directly into uv light, it’s like looking at the sun
Also uv light burns the skin
NOTE: for the remainder of the course search Google under "Ashern Gems"